how do you find the number of electrons
Questions and Answers
How many protons, electrons and neutrons are in an atom of krypton, carbon, oxygen, neon, silver, gilt, etc...?
To find the number of protons, electrons and neutrons in an atom, just follow these easy steps:
Pace 1 - Gather Information
The first matter you will demand to do is discover some data near your chemical element. Go to the Periodic Table of Elements and click on your element. If it makes things easier, you can select your element from an alphabetical listing.
Use the Table of Elements to find your element's diminutive number and atomic weight. The diminutive number is the number located in the upper left corner and the atomic weight is the number located on the bottom, every bit in this instance for krypton:
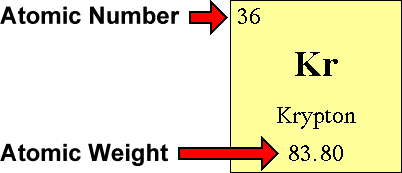
Step 2 - The Number of Protons is...
The atomic number is the number of protons in an atom of an chemical element. In our example, krypton's atomic number is 36. This tells united states of america that an atom of krypton has 36 protons in its nucleus.
The interesting thing here is that every cantlet of krypton contains 36 protons. If an atom doesn't have 36 protons, it can't be an atom of krypton. Calculation or removing protons from the nucleus of an cantlet creates a different chemical element. For example, removing one proton from an atom of krypton creates an cantlet of bromine.
Pace three - The Number of Electrons is...
By definition, atoms accept no overall electrical charge. That means that there must exist a rest between the positively charged protons and the negatively charged electrons. Atoms must have equal numbers of protons and electrons. In our example, an atom of krypton must comprise 36 electrons since it contains 36 protons.
Electrons are arranged around atoms in a special way. If you lot need to know how the electrons are arranged effectually an cantlet, take a look at the 'How do I read an electron configuration table?' page.
An atom tin gain or lose electrons, condign what is known every bit an ion. An ion is nothing more than than an electrically charged cantlet. Adding or removing electrons from an cantlet does not change which element information technology is, just its cyberspace charge.
For case, removing an electron from an cantlet of krypton forms a krypton ion, which is usually written as Kr+. The plus sign means that this is a positively charged ion. It is positively charged considering a negatively charged electron was removed from the atom. The 35 remaining electrons were outnumbered by the 36 positively charged protons, resulting in a accuse of +i.
Step 4 - The Number of Neutrons is...
The atomic weight is basically a measurement of the full number of particles in an atom's nucleus. In reality, it isn't that clean cut. The atomic weight is actually a weighted average of all of the naturally occurring isotopes of an element relative to the mass of carbon-12. Didn't understand that? Doesn't thing. All you really need to find is something called the mass number. Unfortunately, the mass number isn't listed on the Table of Elements. Happily, to find the mass number, all you demand to do is round the atomic weight to the nearest whole number. In our example, krypton'south mass number is 84 since its diminutive weight, 83.80, rounds up to 84.
The mass number is a count of the number of particles in an atom's nucleus. Recall that the nucleus is fabricated up of protons and neutrons. Then, if nosotros want, we tin can write:
Mass Number = (Number of Protons) + (Number of Neutrons)
For krypton, this equation becomes:
84 = (Number of Protons) + (Number of Neutrons)
If we only knew how many protons krypton has, we could figure out how many neutrons information technology has. Wait a minute... Nosotros do know how many protons krypton has! We did that back in Step ii! The atomic number (36) is the number of protons in krypton. Putting this into the equation, we get:
84 = 36 + (Number of Neutrons)
What number added to 36 makes 84? Hopefully, you said 48. That is the number of neutrons in an atom of krypton.
The interesting affair here is that calculation or removing neutrons from an atom does not create a unlike element. Rather, it creates a heavier or lighter version of that chemical element. These different versions are chosen isotopes and most elements are really a mixture of dissimilar isotopes.
If you could grab atoms of krypton and count the number of neutrons each ane had, you lot would find that most would have 48, others would take 47, some would have 50, some others would take 46, a few would have 44 and a very few would have 42. Y'all would count different numbers of neutrons because krypton is a mixture of six isotopes.
In Summary...
For any element:
Number of Protons = Atomic Number
Number of Electrons = Number of Protons = Atomic Number
Number of Neutrons = Mass Number - Atomic Number
For krypton:
Number of Protons = Atomic Number = 36
Number of Electrons = Number of Protons = Atomic Number = 36
Number of Neutrons = Mass Number - Atomic Number = 84 - 36 = 48
Related Pages:
Source: https://education.jlab.org/qa/pen_number.html
Posted by: ottmanpreal1956.blogspot.com

0 Response to "how do you find the number of electrons"
Post a Comment